Abstract
Background: Neutropenic fever is a life-threatening condition which is common in patients with hematologic malignancies, and prompt initiation of appropriate antibiotics is of great importance.Empirical treatment includes monotherapy with a beta-lactam containing anti-pseudomonal activity. Theoretically, time-dependent antibiotics are most active when administered as a continuous infusion, but evidence is lacking in cases of febrile neutropenia. This study examines the optimal dosing regimen (bolus infusion vs. prolonged 4 hour-infusion) for high risk patients undergoing hematopoietic cell transplantation or patients with acute leukemia and febrile neutropenia.
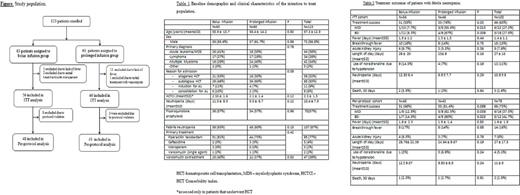
Methods: This was a single center, prospective, unblinded randomized study, of 123 hospitalized hematologic patients enrolled between July 2015 and March 2017. The primary outcome was a successful response to treatment, defined as the combination of- defervescence at 96 hours after initiation of broad spectrum beta-lactams (pipracillin/tazobactam or ceftazidime) or before, sterile cultures after 72-96 hours, and no requirement of additional aminoglycoside therapy after 48 hours. Secondary outcomes included breakthrough fever or additional infection after 5 days from initiation of treatment, death within 30 days of study enrollment, duration of hospitalization, duration of neutropenia, occurrence of renal failure (doubling of serum creatinine levels) within 5 days, and requirement for noradrenaline due to hypotension. Patients were randomized using a computerized random number program and allocation was concealed by sequentially numbered sealed and opaque envelopes. All patients signed informed consent and the study was approved by the Tel Aviv Medical Center Institutional Review Board.
Results: 123 patients were randomized to bolus infusion (n=63) and prolonged infusion (n=60). Febrile neutropenia occurred in 87% of the patients, and these patients were eligible for the intention-to-treat analysis (ITT). 73% of the patients were given at least one dose of antibiotic according to the allocation arm and were eligible for the per-protocol analysis, Figure. The patients' baseline characteristics were well-balanced between the 2 groups, Table 1. In the ITT analysis (Table 2), there was a higher success rate in patients allocated to the prolonged infusion arm, compared to those allocated to the bolus infusion arm (76% vs. 55%, p= .03). This was also true for the subgroups of patients with microbiologically documented infections (MDI) - (55.6% vs. 7.7%, p=.013) and blood stream infections, (BSI) - (67% vs. 8.3%, p=.009). Similarly, in the per-protocol analysis, the success rate was higher in patients allocated to the prolonged infusion arm who had MDI (62.5% vs. 12.5%, p=.039) or BSI (80% vs. 14.3%, p=.023). There was no difference in the secondary endpoint outcomes between the 2 groups. 30-day death rate was similarly low in both groups. Prolonged beta-lactam infusion was associated with significantly higher treatment success in a logistic regression model that included either BSI (odds ratio of 2.4, 95% CI 0.97-6.1, p=.058) or MDI (OR 2.8, 95% CI 1.1-7.4, p=.032) .
Conclusions: This is the first randomized study showing superior treatment outcomes in patients with high-risk febrile neutropenia treated with prolonged versus bolus beta-lactam infusion. Optimization of beta-lactam dosing is a widely available and cost-effective strategy to improve treatment outcomes in patients with high risk febrile neutropenia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract